Molecular Orbital Diagram For S2
Molecular orbital diagram carbon monoxide orbitals configuration energy level electronic chemistry mo theory bonding explanation bond fluorine nitrogen questions oxygen Molecular orbital theory Molecular orbital theory
Electrons | Biology for Majors I
Orbital molecular diagram cl2 s2 molecule orbitals electron bond unpaired bonding c2 molecules diatomic energy theory valence electrons chlorine li2 Bonding molecular between antibonding orbital orbitals mo bonds theory difference covalent pi diagram electron energy ethylene chemistry polyatomic anti multiple Atomic theory
38 o2 2- molecular orbital diagram
Molecular orbitals bond order bonding electrons chemistry ion has unpaired geometry delocalized chemwiki exercises structure answers general principles v1 covalent1. overview of most basic ao. 1s, 2p and 3d molecular orbitals shown in Orbital molecular n2 orbitals bond diatomic valence o2 atomic homonuclear atoms sigma molecule majors below chem cnx will shown verticalOrbitals atomic atom structure electron shapes sublevels energy chemistry modern elements shape electrons model levels sub level theory configurations periodic.
Chapter 6.5 delocalized bonding and molecular orbitalsBond order molecular orbital theory magnetic chemistry properties strength Orbital molecular theory diagram libretexts chemistry energy chem bond electrons order unpaired bonding deki revision apiWhat is the difference between bonding and antibonding molecular.

Chapter 6.5 delocalized bonding and molecular orbitals
Orbital molecular diatomic molecules diagram chemistry theory orbitals diagrams energy bond bonding level electron cl2 libretexts second delocalized homonuclear rowOrbitals molecular 2p shown coordinates Solved consider the partially filled-in molecular orbitalMolecular orbitals in carbon monoxide co.
8.4: molecular orbital theoryOrbital molecular mo o2 diagram theory orbitals bond oxygen order paramagnetic configuration energy electrons unpaired diagrams two lone draw which Need a molecular orbital diagram for s2, s2+ and s2- not theDraw the molecular orbital diagrams for the following diatomic.

Orbital study calculation
Chemistry 101: molecular orbital theory, bond order, bond strengthMolecular orbital diagram bn mo orbitals bond diagrams order cl2 theory paramagnetic energy level draw bonding valence electrons chemistry homonuclear Molecular orbital electrons 3p 3s chegg partially consider filledOrbital molecules diatomic polyatomic diagrams ions rank rm eq align dfrac.
Molecular orbital theoryElectrons subshells biology orbitals electron figure shaped 2.4: molecular orbital theoryOrbital molecular o2 ozone paramagnetic molecule bonding libretexts orbitals valence molecules chem.


Molecular Orbital Theory

Atomic Theory - Honors Chemistry

Solved Consider the partially filled-in molecular orbital | Chegg.com

What is the difference between bonding and antibonding molecular

Molecular Orbital Theory | Grandinetti Group
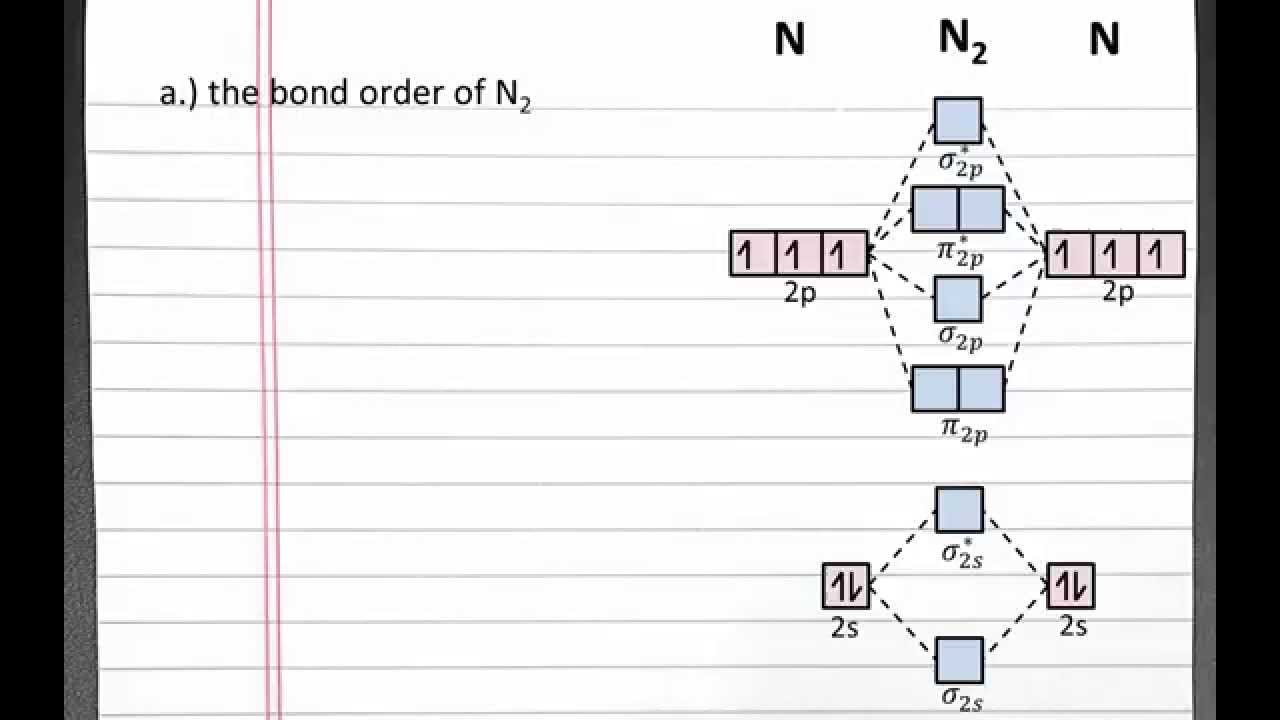
CHEMISTRY 101: Molecular Orbital Theory, Bond order, bond strength

38 o2 2- molecular orbital diagram - Wiring Diagram Info

2.4: Molecular Orbital Theory - Chemistry LibreTexts

1. Overview of most basic AO. 1s, 2p and 3d molecular orbitals shown in